|
| Polyphosphate fluid fertilizer |
Phosphorus deficiency limits the growth and
productivity of plants in many parts of the world. Since many soils are low in
P, this nutrient is commonly added to improve crop yield and quality.
Phosphorus is derived from geologic deposits distributed across the globe.
Polyphosphate is an excellent liquid fertilizer that is widely used in
agriculture.
Production
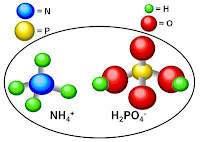
Phosphoric acid is the starting material for most commercial phosphate
fertilizers. However, the acidity and some of the chemical properties make
this material difficult to use directly. When phosphoric acid and ammonia are
reacted, water is driven off and individual phosphate molecules begin to link
together to form a “polyphosphate” fluid fertilizer.
A single phosphate molecule is called orthophosphate. “Poly” refers to
multiple phosphate molecules linked in a chain. Each linkage of phosphate
molecules has a name depending on its length, although polyphosphate is the
general term that includes all of these linked molecules.
The most common ammonium polyphosphate
fertilizers have N-P2O5-K2O
composition of 10-34-0 or 11-37-0. Polyphosphate fertilizers offer the
advantage of a high nutrient content in a clear, crystal-free fluid that is
stable under a wide temperature range and has a long storage life. A variety of
other nutrients mix well with polyphosphate fertilizers, making them an
excellent carrier for micronutrients that may be needed by plants.
 |
| "Poly" in polyphosphate refers to chains of phosphate... short or long |
Chemical Properties
Agricultural Use
In polyphosphate fertilizer,
between half and three-quarters of the P is present in chained polymers. The
remaining P (orthophosphate) is immediately available for plant uptake. The
polymer phosphate chains are primarily broken down to the simple phosphate
molecules by enzymes produced by soil microorganisms and plant roots. Some of
the polyphosphate will decompose without the enzymes. The enzyme activity is
faster in moist, warm soils. Typically, half of the polyphosphate compounds are
converted to orthophosphate within a week or two. Under cool and dry
conditions, the conversion may take longer.
Since polyphosphate
fertilizers contain a combination of both orthophosphate and polyphosphate, plants
are able to use this fertilizer source very effectively. Most P-containing
fluid fertilizers have ammonium polyphosphate in them. Fluid fertilizers are
commonly used in production agriculture, but not widely used by homeowners.
Fluids are convenient for farmers since they can be easily blended with many
other nutrients and chemicals and each drop of fluid is exactly the same. For
most situations, the decision to use dry or fluid fertilizers is based on the
price of nutrients, fertilizer-handling preferences, and field practices rather
than significant agronomic differences.
Management
Practices
Ammonium polyphosphate is
primarily used as a source of P nutrition for plants. Since P has limited
mobility in most soils, efforts should be made to place the material as close
to developing roots as practical. Practices should be adopted to minimize the
movement of P from the soil into adjacent water. Excess P in surface water can
stimulate the growth of undesirable algae.
Non-agricultural
Use
Phosphate is an essential
component in human nutrition. Polyphosphate is an approved additive for food
and requires no special precautions in handling. Polyphosphate compounds are
widely used as a flame retardant on many products, including wood, paper,
fabric, and plastic. It is also used as a commercial retardant for forest
fires. The mode of action involves the ammonium polyphosphate forming a charred
layer after burning, thereby preventing further flames.
Read the fact sheet on some of the important properties of polyphosphate and how it is used.
http://tinyurl.com/polyphosphate