 |
Massive machines are used to recover phosphate rock from Florida |
Maintenance of an adequate phosphate
supply in the soil is essential for sustaining global food supplies. Many
soils need an additional source of phosphate to supplement the native supply in
order to meet this minimum requirement. Crops remove relatively large amounts
of phosphate from the soil in the harvested portion. At some point, it is
necessary to replenish the supply of this nutrient.
Early sources of P were limited to animal manure, which did not supply any new nutrients, but merely allowed them to be transported from one area to another. The first commercial fertilizer became available when it was discovered that adding acid to animal bones would chemically unlock the phosphate and make it available for plant uptake.
Early sources of P were limited to animal manure, which did not supply any new nutrients, but merely allowed them to be transported from one area to another. The first commercial fertilizer became available when it was discovered that adding acid to animal bones would chemically unlock the phosphate and make it available for plant uptake.
 | |
| Early advertisement for superphosphate |
Phosphate rock is the raw material now used in commercial fertilizer production. Phosphate rock is extracted from the earth in many countries. Most of the phosphate rock is used for fertilizer production, with smaller amounts going to various industrial uses. Although phosphate rock is a limited natural resource, at current rates of use the world phosphate rock reserves and resources should be adequate for the foreseeable future.
Phosphate rock is generally extracted with surface mining techniques and
then the pit is later filled, re-vegetated, and reclaimed. The quality of
the rock will vary depending in the level of naturally occurring impurities.
The rock is screened and crushed to prepare it for processing with a source of
acid. After the phosphate rock has reacted with acid, the soluble phosphate is
transformed into many common fertilizers and transported across the world. The
largest users of phosphate fertilizers are China and India.
Phosphate has many important functions in plants. Perhaps the most noted roles are in photosynthesis, respiration, energy storage and transfer, cell division, and cell enlargement. Adequate phosphate also promotes early root formation and growth.
Plants absorb most of their P as the primary orthophosphate ion (H2PO4-). Smaller amounts of secondary orthophosphate ion (HPO42-) are taken up. Other forms of P can be utilized, but in much smaller quantities than orthophosphate.
There are many excellent sources of phosphate fertilizer. The selection of a particular product depends on price, physical characteristics, and nutrients accompanying the phosphate. Agronomic studies have shown that there is no significant difference in plant response to common phosphate fertilizers if they are used properly. The most common fertilizers include:
• Diammonium phosphate (DAP) – DAP is the world’s most widely used P fertilizer. It is made from two common constituents in the fertilizer industry and it is popular because of its relatively high nutrient content and its excellent physical properties.
Phosphate has many important functions in plants. Perhaps the most noted roles are in photosynthesis, respiration, energy storage and transfer, cell division, and cell enlargement. Adequate phosphate also promotes early root formation and growth.
Plants absorb most of their P as the primary orthophosphate ion (H2PO4-). Smaller amounts of secondary orthophosphate ion (HPO42-) are taken up. Other forms of P can be utilized, but in much smaller quantities than orthophosphate.
There are many excellent sources of phosphate fertilizer. The selection of a particular product depends on price, physical characteristics, and nutrients accompanying the phosphate. Agronomic studies have shown that there is no significant difference in plant response to common phosphate fertilizers if they are used properly. The most common fertilizers include:
• Diammonium phosphate (DAP) – DAP is the world’s most widely used P fertilizer. It is made from two common constituents in the fertilizer industry and it is popular because of its relatively high nutrient content and its excellent physical properties.
 |
| Molecular structure of diammonium phosphate |
 | |
| Diammonium phosphate fertilizer |
• Monoammonium phosphate (MAP) – A widely used source of P and N, it
is made of two constituents common in the fertilizer industry. MAP has the
highest P content of any common solid fertilizer.
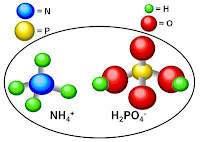 |
| Molecular structure of monoammonium phosphate |
 |
| Monoammonium phosphate fertilizer |
• Ammonium polyphosphate (APP) – When phosphoric acid and ammonia are
reacted, water is driven off and individual phosphate molecules begin to link
together to form a polyphosphate fluid fertilizer.
 |
| Polyphosphate fluid fertilizer |
• Triple superphosphate (TSP) – TSP was one of the first high
analysis P fertilizers that became widely used in the 20th century. It is an
excellent P source, but its use has declined as other P fertilizers have become
more popular.
 |
| Triple superphosphate |
The use of regular soil testing and consultation with a local certified crop
adviser will provide guidance on how to best manage the phosphate supply for
your crops. The next time you apply phosphate fertilizer, consider the
complex journey that it took to get those nutrients to your plants.
A visual tour of the phosphate production process can be seen at this URL: http://info.ipni.net/phosphatetech
A visual tour of the phosphate production process can be seen at this URL: http://info.ipni.net/phosphatetech
Thank you for providing clear information on this. you can also refer Water Soluble Fertilizer.
ReplyDelete